Quality is the root of company survival and development. To improve our quality management level,build a good company view and match international quality management standard.IPE Platic & Hardware Co.,LTD (Zhanwei) have completed this "quality manual" according to the requirement of GB/TI9001-2000 idt ISO9001:2000.
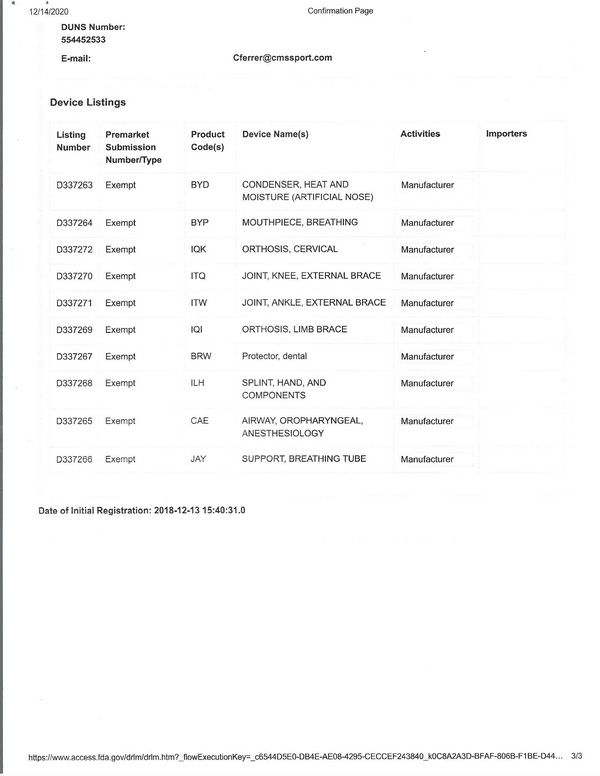
Since 2018, IPE has gained the FDA certificate. The registration number is 3015142701 and the DNS number is 554452533.
Customer satisfaction is extremly important to us. We take pride in providing you with high quality controlled products. From raw material inspection to final packaging check off, we can guarantee our products will follow our rigorous quality control process. We produce high volume custom and self-made products on a daily basis and quality control is upheld from start to finish.
Design input and engineering development ensures quality processing throughout the entire manufacturing operation
Continually upgrading our machining and fabrication equipment to ensure high quality
Quality control with the Ministry of 3d MM projectors, color device, micrometer, temperature measurement, data caliper, height device, the thickness instrument and equal sophisticated electronic detection equipment for the detection precision products provide effective technical support. Products manufactured layers of control, professionals and powerful IQC, QA, IPQC lineup for the customers to provide complete, accurate quality assurance.
You are the most important aspect of our company and we do our best to keep you satisfied!
Quality Certification
Besides finding our product's and component's benefit during your daily life, a set of strict quality & environmental management system has been implementing at all production processes in order to ensure the superior product quality. In IPE factory, high quality products are born-in and you don't need to ask for. All level of staff and workers are equipped with this mind set explicitly. Teamwork, Professional, Innovation and Integrity are also our requirements to all staff. We always work on benchmarking against the international practices and standards under the award of ISO9001:2008,ISO14000 Quality & Environmental Management System Certificate.

FDA_confirmation_registration